 |
| August 24, 2021 | Volume 17 Issue 32 |
Designfax weekly eMagazine
Archives
Partners
Manufacturing Center
Product Spotlight
Modern Applications News
Metalworking Ideas For
Today's Job Shops
Tooling and Production
Strategies for large
metalworking plants
Researchers figure out how to combine almost any metals
Researchers at ETH, a public university in Zurich, Switzerland, have produced nanocrystals made of two different metals using an amalgamation process whereby a liquid metal penetrates a solid one. This new and surprisingly intuitive technique makes it possible to produce a vast array of intermetallic nanocrystals with tailored properties for diverse applications.
Nanocrystals are nanometer-sized spheres consisting of regularly arranged atoms. Owing to their advantageous properties, they are on the rise in several technologies. Semiconductor nanocrystals, for instance, are used in new-generation television screens. More recently, so-called intermetallic nanocrystals, in which two different metals combine to form a crystal lattice, have made a name for themselves as they promise improved and unique applications. Those applications range from catalysis to data storage and medicine.
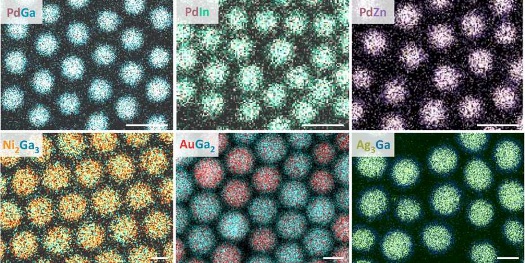
Intermetallic nanocrystals (electron microscope images) made from different combinations of metals. The white bar indicates 10 nanometers. [Images: Chemistry and Materials Design group, ETH]
In theory, there are tens of thousands of possible combinations of metals that could make up such nanocrystals, with a correspondingly large number of different material properties. So far, however, it has only been possible to make nanocrystals out of a few such pairings. A team of researchers at ETH Zurich, led by Maksym Yarema and Vanessa Wood at the Institute for Electronics, has now developed a new technique that, in principle, allows one to realize nearly all possible combinations of intermetallic nanocrystals. Their results were recently published in the scientific journal Science Advances.
Surprisingly intuitive and simple method
"Our method is simple and intuitive -- so intuitive, in fact, that we were surprised that no one had had this idea before us," says Yarema. In conventional procedures for producing nanocrystals made of a single metal, the metal atoms are introduced in molecular form, for instance as salts, into a solution in which the nanocrystals then form. "Theoretically, that can also be done with two different metals, but in practice it is difficult, or even impossible, to combine distinctly dissimilar metals in the reactor," Yarema explains. Therefore, the ETH scientists resorted to a procedure that has been used for centuries: amalgamation, a particular kind of fusing or blending metals.
Liquid metals
Amalgams are particularly well known from dentistry, where they are used as a filling material, and also from gold mining. In both cases, liquid mercury is added to dissolve other metals (for tooth fillings, a mixture of copper, zinc, and silver). However, amalgamation also works with any other liquid metal. Besides mercury, which is liquid even at room temperature, there are a number of metals with relatively low melting points, such as gallium (30 C melting point), indium (157 C), or tin (232 C).
Amalgamation approach for nanocrystals
Yarema and his colleagues use the amalgamation approach at the nanoscale. The reaction starts with the dispersion of nanocrystals containing a single metal, for instance silver. Then, the atoms of the second metal -- say, gallium -- are added in molecular form (in this case as amides, a compound of carbon, hydrogen, and nitrogen), while the mixture is heated to around 300 C.
Initially, the high temperature causes the chemical bonds in the gallium-amide to break up, allowing liquid gallium to accumulate on the silver nanocrystals. Now, the actual amalgamation process begins, during which liquid gallium creeps into the solid silver. Over time, a new crystal lattice is formed, in which eventually silver and gallium atoms are regularly arranged. Then everything is cooled down again, and after 10 minutes the nanocrystals are ready.
"We are amazed how efficient the amalgamation is at the nanoscale. Having one liquid metal component is the key to fast and uniform alloying within each nanocrystal," says Yarema.
Totally controllable process
Using the same technique, the researchers have already produced different intermetallic nanocrystals such as gold-gallium, copper-gallium, and palladium-zinc. The amalgamation process itself can be precisely steered. Through the number of secondary atoms, introduced into the solution as amides, the proportion of the metals in the nanocrystals can be accurately controlled. Taking the example of gold-gallium (chemical symbols Au and Ga), the researchers have shown that in this way nanocrystals with very different proportions can be produced, such as 1:2 (AuGa2), 1:1 (AuGa), or 7:2 (Au7Ga2). The size of the final intermetallic nanocrystals can also be predicted precisely from the size of the initial nanocrystals and the increase in size due to the second metal.

The production process of an intermetallic nanocrystal (upper row: schematic, lower row: electron microscope images). To the solution containing nanocrystals of the first material (left), the second metal ("M") is added as an amide and subsequently accumulates as a liquid on the nanocrystals (center). Amalgamation finally results in intermetallic nanocrystals (right). [Images: CMD, ETH]
Tailored nanocrystals for applications
The researchers anticipate great potential for technological applications due to the exact controllability of the composition and size of the nanocrystals together with the possibility to combine the metals almost at will. "Because the amalgamation synthesis of nanocrystals enables so many new compositions, we cannot wait to see them at work in improved catalysis, plasmonics, or lithium-ion batteries," says Yarema. Catalysts made of nanocrystals, for instance, can be precisely tailored and optimized for a particular chemical process that they are supposed to accelerate.
Source: ETH Zurich
Published August 2021
Rate this article
View our terms of use and privacy policy
